New approach may yield modified messenger RNAs for treating a wide range of conditions
The LEGO framework could help scientists create mRNA therapies that last longer and require smaller doses.
The life-saving use of messenger RNA (mRNA) in COVID-19 vaccines was a public example of the potential of mRNA-based therapies, which hold great promise for a wide range of treatment applications from cancer immunotherapy to gene editing.
Working toward a systematic method of optimizing mRNA drugs for specific uses, researchers at the Broad Institute of MIT and Harvard and the Massachusetts Institute of Technology have developed an approach for tailoring mRNAs to produce a greater abundance of protein, or to produce protein for a longer period of time, compared to native mRNA. This opens the door to delivering mRNA-based therapies at lower doses with fewer side effects for a variety of conditions.
Building off previous research that investigated attaching multiple chemical “tails” to mRNA, the researchers, led by Broad core institute member and Thomas D. and Virginia Cabot Associate Professor of Chemistry Xiao Wang, systematically tested many different chemical modifications of mRNA and measured their effects on protein translation. Using what they learned from these experiments, they developed a framework — ligation-enabled messenger RNA-oligonucleotide assembly, or LEGO — that enables researchers to chemically modify the structure of mRNA molecules and influence their interactions with the cell’s protein-translation machinery, achieving desired therapeutic effects. Their work on LEGO is published in Nature Biotechnology.
“The overarching goal of our project is to create treatments that utilize mRNA’s full potential as an informational molecule that can deliver any protein of interest,” said Hongyu Chen, a PhD student in Wang’s lab and co-first author on the study with fellow PhD students Dangliang Liu and Abhishek Aditham. “It’s a very generalizable technology.”
The team hopes to ultimately create a comprehensive protocol that would allow researchers to optimize every component of an mRNA drug, achieving therapeutic control previously only possible in more traditional small molecule drugs.
“We ultimately want to synthetically expand the alphabet, create new structures, and decode the chemical language of mRNA medicine to maximize its potential under different therapeutic settings,” said Wang, who is the senior author on the paper and also the Thomas D. and Virginia Cabot Associate Professor of Chemistry at MIT.
Protein boost
The mRNA vaccines developed for COVID-19 only need to generate a modest amount of protein in order for the immune system to kick in and develop robust antibodies against infection. But in some conditions, such as hemophilia or diabetes, patients lack or have low levels of a protein, hormone, or enzyme, and require therapies that can replace them in the body. In their natural state, mRNA molecules are short-lived and typically translated into protein only for a brief period of time before degrading. But depending on the therapeutic goal, an ideal mRNA-based treatment might produce protein for a long time, reducing the number of times a patient would have to receive treatment, or provide a large amount of protein quickly.
This is where LEGO could become incredibly beneficial.
LEGO allows researchers to optimize mRNA molecules for translational efficiency by altering their 5′ and 3′ ends — their “caps” and “tails,” respectively. Modifying an mRNA’s cap can affect how well mRNA gets translated into protein, while modifications to the tail impact mRNA stability and degradation. By mixing and matching cap and tail modifications — including adding multiple caps and tails branching off the molecule — Chen, Liu, Aditham, Wang, and their colleagues found they could fine tune mRNAs’ longevity and translational efficiency to achieve specific therapeutic goals.
The researchers used LEGO to develop an mRNA hormone replacement therapy encoding the hormone erythropoietin, which stimulates red blood cell production to treat anemia. In mice, the drug — an mRNA with a dual-branched cap and other modifications — caused cells to produce eight times more protein than regular mRNA. They also created an optimized COVID-19 vaccine, using the same mRNA modifications, that in mice triggered a 17-fold increase in antibody production compared to a regular COVID-19 vaccine after two weeks.
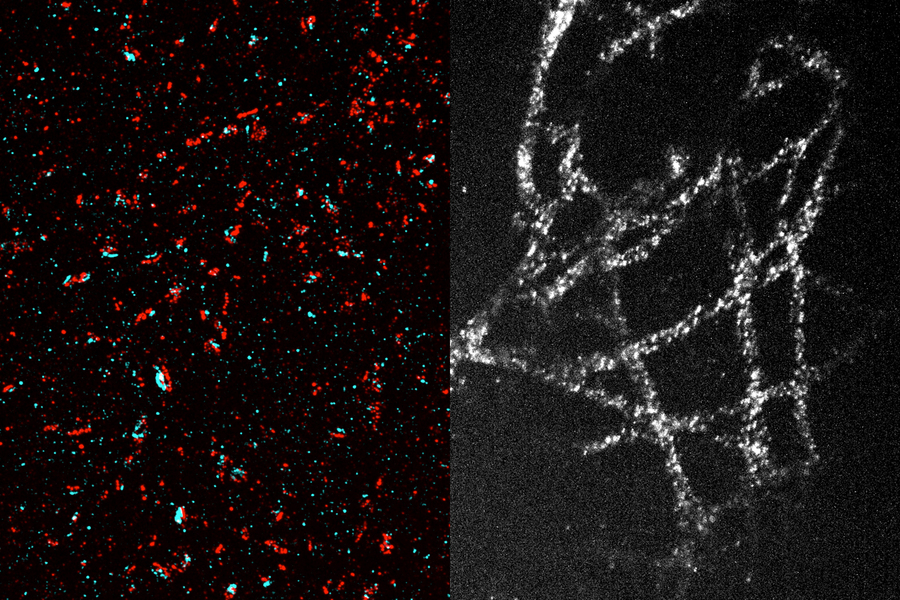
In addition to optimizing linear mRNA, the team also used LEGO to test modifications of circular mRNA, which is more resistant to degradation but which the cell translates using a less efficient method. By adding a branched cap to circular RNA, the researchers created a “QRNA” that, when designed to encode a fluorescent protein, produced up to a 60-fold increase in fluorescence compared to regular circular RNA. While QRNA is further than linear mRNA from becoming an effective therapeutic approach, the researchers were able to learn more about how cells produce protein naturally by observing how different modifications affected QRNAs’ translation.
The results show mRNA’s potential for developing highly effective and targeted treatments for a wide range of conditions while providing a framework for constructing mRNAs tailored for specific needs.
“In most of the applications that we have already tried — in vaccines or in protein replacement therapeutics — we can achieve a much higher therapeutic effect with these modifications,” said Liu. “Such a tiny chemical change can contribute such an amazing increase to mRNA stability and translatability.”